Production systems for radiopharmaceutical manufacturing





Designed for reliable scale-up, regulatory alignment, and smooth tech transfer — trusted by leading radiopharmaceutical manufacturers.
Cody’s involvement in production equipment for radiopharmaceuticals spans more than 15 years beginning with an early collaboration with Algeta (later acquired by Bayer). Cody developed and built custom-engineered machines and equipment for use in radiochemical processes within hot cells, as well as for product dispensing and packing. These early systems were designed with a strong focus on operator safety, repeatability, and compliance with GMP requirements.
Our experience in radioactive isotope processing, and the strict regulatory conditions within that space, directly informs how we design production technology and manage risk, validation, and operational reliability.
“Cody has demonstrated exceptional competence in engineering complex, high-quality systems. Their deep understanding of radiopharmaceutical processes and strong execution capabilities make them a trusted partner in our mission to industrialize Actinium-225 production.” – Samy Bertrand, Chief Technology Officer, PanTera
Today, Cody is a trusted partner in innovative production technology for radiopharmaceuticals, used by leading companies that depend on GMP-compliant machinery delivering reliable performance under demanding conditions. We design and build solutions for pre-clinical, clinical, and commercial production.
Over many years, Cody has delivered advanced solutions for the handling of radioisotopes and radiopharmaceutical products, in close collaboration with leading players in the industry. This experience provides a strong foundation for taking full responsibility for complete radiopharmaceutical projects—from initial concept to commercial production.

We follow the entire project lifecycle, from early dialogue and process clarification, through development and manufacturing, to testing, validation, and documentation in accordance with relevant regulatory requirements (CE, FDA, EudraLex, GAMP5, etc.). When requirements are high and margins for error are minimal, Cody delivers equipment designed for the realities of radiopharmaceutical production. Our solutions are built for precision, safe operation, and reliable performance in controlled environments such as hot cells - developed in close partnership with our customers.
Our Services
Pre-clinical phase
We develop enabling technology for handling isotopes with low radiation doses during the pre-clinical phase, supporting verification of chemistry, processes, and production methods.
Clinical phase
We advance the technology to higher radiation doses and increased production volumes, with a higher level of automation.

Commercial phase
We industrialise the technology and develop high-technology systems for commercial production - whether radiochemical processing in hot cells or automated packaging, labelling, and packing of radiopharmaceuticals for distribution.
As a full-service provider of tailored solutions, we adapt technology to each customer’s specific process, technology, and requirements. We take pride in understanding our customers’ goals and deliver the greatest value through early involvement.

Cody carries out R&D projects, pre-projects, feasibility studies, and prototyping to ensure technical and regulatory viability before full project execution.
Selected Projects
The Way We Work
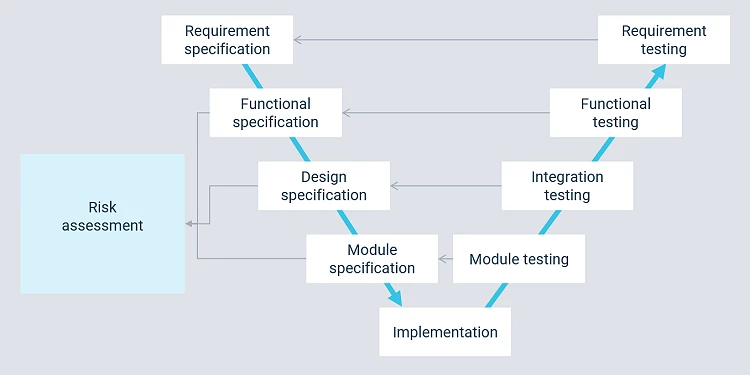
Preliminary projects and studies are part of a larger development process on the way to a validated solution. Cody uses the V-model in our project work.

The Cody Team

Experienced engineers shaped by real-world radiopharma production
Over many years of hands-on work in the field, the Cody team has built a unique understanding of the demands of radiopharmaceutical production. Our engineers know the challenges that arise within hot cells, in sterile environments, and when handling complex isotopes — and they know how to solve them.
This insight allows us to quickly identify technical limitations, uncover opportunities, and develop a solution that truly fits your needs, not just on paper.
Whether it’s custom engineering, integration into existing lines, or entirely new production setups, we combine solid problem-solving with deep process understanding to deliver a result that works in practice - every time.
Cross-disciplinary expertise throughout the entire project
Our team consists of engineers, automation specialists, designers, and validation experts who understand the full production ecosystem — and they stay involved through the entire project lifecycle, from early concept and design to FAT, SAT, and operator training.


Magnus is our Chief Business Development Officer, and with his experience in both mechanical design, automation, project management, and sales, he has full control over what Cody can deliver. He also delves deep into the Machinery Directive, CE marking, and harmonized standards to ensure that what we deliver complies with laws and regulations.
Read more about our Radiopharma projects
Answers to the Most Common Questions
What safety and quality certifications does Cody hold?
All systems are CE-marked, built under Cody’s ISO 9001-certified quality management system, and comply with EU radiation safety and machine directive requirements.
Does Cody work with major pharma and research partners?
Yes. Cody has delivered equipment to Bayer, GE Healthcare, PanTera, and several national isotope production facilities — supporting cutting-edge radiopharmaceutical research and manufacturing across Europe.
What documentation does Cody provide for GMP qualification?
Each project includes a complete documentation package — user requirements (URS), design documentation, FAT/SAT reports, and IQ/OQ protocols.
This ensures that every radiopharma system is fully traceable, auditable, and ready for regulatory inspection.




