Advanced Automation Solutions for Europe’s Pharmaceutical Industry







Reduce validation time. Increase throughput. Ensure compliance.
At Cody, we specialize in delivering customized, high-tech, automated solutions tailored for the pharmaceutical and radiopharmaceutical sectors across Europe. Our decade-long collaborations with industry leaders like Bayer, GE Healthcare, and Vistin Pharma have honed our expertise in material selection, design, functionality, and digital control systems.
Understanding the stringent requirements of the pharmaceutical industry, we emphasize Good Manufacturing Practice (GMP) to deliver fully validated, production-ready systems. Our proficiency ensures compliance with rigorous standards for traceability, batch accounting, and access control, providing you with reliable and efficient solutions.
Where Every Project Begins
Our process typically begins with comprehensive studies or preliminary projects, where we define goals, identify requirements, and perform necessary analyses. This phase is crucial in laying the foundation for successful project outcomes, allowing us to innovate and find optimal solutions tailored to your specific needs.
Partner with Cody to enhance your pharmaceutical production capabilities, ensuring high productivity, superior quality, and efficient resource utilization. Our commitment to innovation and excellence positions us as your ideal collaborator in navigating the complexities of pharmaceutical manufacturing.

Studies / Preliminary Projects:
Most of Cody's deliveries in special machinery and product development begin with a study or a preliminary project. The preliminary project phase is a crucial part of the development process, as it lays the foundation for a successful project by defining goals, identifying requirements, and performing necessary analyses. It is in the studies and preliminary projects that Cody has its greatest innovations and the greatest potential to find the optimal solutions for clients. We find that the earlier we are involved in a project, the better the end result will be. A preliminary project at Cody may include the following key elements:
Define Goals and Scope
What should the machine/product achieve, and what specific needs should it address? Define the overall requirements for the solution.
Creative Phase
Cody's developers work creatively with several potential solutions and methods before presenting a recommended solution.
Requirement Specification
Identify functional and non-functional requirements. Involve stakeholders, including operators, engineers, and management, to ensure that all needs and expectations are captured.
Risk Analysis
Identify possible risks related to technology choices, project execution, and operation.
Design Specification
Develop a concept design that provides a rough sketch of the machine's layout and functionality.
Offer for Detailed Design, Construction, and Delivery of Solution
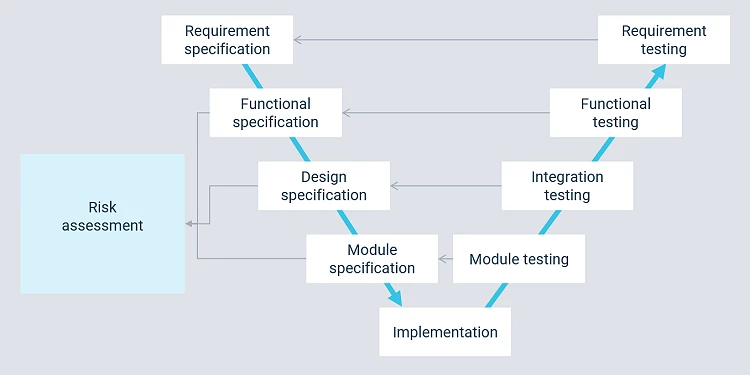
The way forward
Preliminary projects and studies are parts of a larger development process on the path toward a validated solution. Cody uses the V-model in our project work.

Radiopharmacy
Cody has worked closely with the radiopharmaceutical industry for many years and has contributed to most stages of development and production. From basic research on manufacturing processes, through custom-designed hot cell and lab equipment, to packaging and full-scale GMP production and packaging machinery. We also produce equipment with integrated shielding made of materials such as tungsten, lead, or lead glass.
Autoclave tray
We design and deliver trays for autoclaving vials, syringe sleeves, instruments, and more. We also produce accessories such as carts and racks.

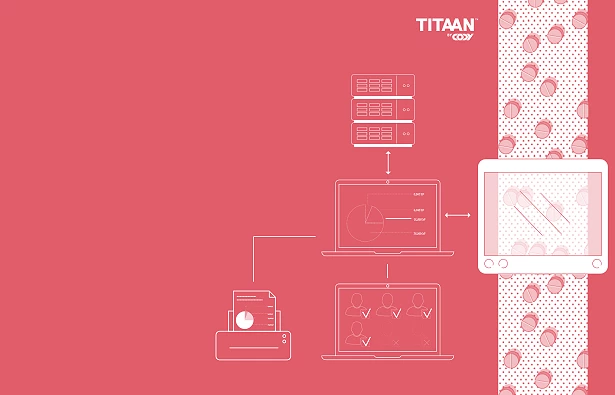
Validated Data Capture
In the development of production machinery for the pharmaceutical industry, Cody has identified the need for a digital solution that collects and stores data from both new and existing machines and makes these available to various IT platforms. In short, it serves as "the missing link" between the production machines and the company's overall IT system. Therefore, we have developed our own software called TITAAN, which meets this need and fulfills the industry requirements for pharmaceutical production (GAMP5, 21 CFR Part 11).
TITAAN can capture data from production in several different ways. All data is timestamped and securely stored, making it available for automatic reporting, display in dashboards, export, or search. Additionally, the software handles parameter changes and user actions (audit trail), electronic signatures, recipes, alarm logs, and reports. TITAAN is currently used by companies such as Bayer, ABB, and TechnipFMC.

Magnus is our Chief Business Development Officer, and with his experience in both mechanical design, automation, project management, and sales, he has full control over what Cody can deliver. He also delves deep into the Machinery Directive, CE marking, and harmonized standards to ensure that what we deliver complies with laws and regulations.
Latest in Pharma
Other industries
Radiopharma
Find out why Cody is a trusted provider of production solutions for the radiopharma industry
Radiopharma
Find out why Cody is a trusted provider of production solutions for the radiopharma industryProduction
Cody is a machine builder with extensive experience in developing machines that meet industry needs.
Production
Cody is a machine builder with extensive experience in developing machines that meet industry needs.Process Industry
Automate repetitive tasks to ensure they are done correctly
Process Industry
Automate repetitive tasks to ensure they are done correctlyAssembly and lifting equipment
Not all assembly and lifting operations can be solved with standard equipment
Assembly and lifting equipment
Not all assembly and lifting operations can be solved with standard equipmentTest equipment
We provide custom-built testing and logging equipment tailored to your needs.
Test equipment
We provide custom-built testing and logging equipment tailored to your needs.Startup
Cody is a supplier with extensive experience working with entrepreneurs and startups across various industries.
Startup
Cody is a supplier with extensive experience working with entrepreneurs and startups across various industries.Research
Innovation starts with curiosity. At Cody, research is a cornerstone of how we develop smarter solutions for radiopharma, automation, and advanced...